Linking Angelman and Dup15q Data for Expanded Research (LADDER), is a strategic collaboration between the Angelman Syndrome Foundation and the Dup15q Alliance. Both organizations have made significant investments in the search for therapeutics and treatment.
See a press release for more information.
What is LADDER?
LADDER is a database that brings together information about Angelman and Dup15q syndromes, collected from sources all over the world including:
- The Angelman Natural History Study
- Patient visits to ASF Clinics in the LADDER Learning Network
- Research studies done on Angelman syndrome and Dup15q
- Global Angelman Syndrome Registry
How does LADDER Advance Research
Before LADDER, information about an individual with AS from research studies, doctor visits and registries was stored in disparate systems. There was no connection or way to link a research participant to their information from a clinic visit.
Gathering the information in one place and connecting it, creates a higher level of understanding of AS which increases the potential for future discoveries that will lead to clinical trials and treatments. In addition, we will better understand how development, behavior and clinical needs change over time.
The data in LADDER can be accessed by:
- Physicians who treat Angelman syndrome
- Researchers who are working to find treatments and a cure
- Pharmaceutical partners who are working on drug development projects
Research Projects
The following research was conducted with information obtained through LADDER.
Developmental Skills of Individuals with AS
Anjali Sadhwani, PhD, Boston Children’s Hospital
How you can help
Visit the LADDER website and enroll!
During enrollment, you will be asked questions about your individual. If he/she has participated in a research study or visited an ASF Clinic, some information could already be in LADDER. During enrollment you will consent to linking the information together.
Plus, completing enrollment in LADDER, will reduce the number of times you will need to fill out the same information when participating in collaborating research studies or visiting an ASF Clinic.
Enrollment consists of completing 3 steps:
- A consent form to participate in LADDER.
- A clinical needs questionnaire that gives background information about your family such as age, gender, and genotype.
- A consent to have information received from AS Clinics you may have gone to or from research studies you may have participated in.
On average, enrollment takes 20 minutes.
Additional Information
After reviewing this information, if you still have questions, contact the LADDER team at ladder@rti.org or call 1-888-454-2588.
What is LADDER? Linking Angelman and Dup15q Databases for Expanded Research (LADDER), is a database platform that links data on individuals living with Angelman or Dup15q syndromes. Data is collected from multiple resources, such as such as research studies, registries, caregiver reports, and clinic visits.
How is LADDER different from the Global Angelman Syndrome Registry? The Global Angelman Syndrome Registry is an international program designed to identify and monitor the needs of individuals with Angelman and their families. The registry also collects information on the natural history of Angelman and informs families about eligibility for clinical trials. LADDER is working closely with the Global Angelman Syndrome Registry team to maximize data sharing and minimize burden for families.
How is LADDER different from the Angelman Natural History Study? The Angelman Syndrome Natural History Study is a federally funded research study with the goal of documenting how development, behavior, and medical concerns in children with Angelman syndrome change as they age. Data from the Angelman Natural History study is stored in LADDER and combined with clinic and registry data for those who have enrolled in LADDER.
Why should I enroll in LADDER? By linking multiple sources of information LADDER can expand research and accelerate the development of interventions and treatments for individuals with Angelman or Dup15q and their families.
How long does enrollment take? On average, it takes around 10-15 minutes to complete each form needed to enroll in LADDER. The clinical needs questionnaire was designed so that you are only asked questions about topics relevant to the individual you are completing the questions about; therefore, depending on their unique history and needs these forms may take anywhere from 20-60 minutes to complete. The clinical needs questionnaire is completed (or updated) at least once a year. The questionnaire will also be sent to you ahead of an AS Clinic visit. Once you have completed the questionnaire the first time, subsequent completions will request updates to the previous version.
What if I have already participated in a research study or gone to a clinic? LADDER has partnered with the Angelman Natural History Study, the 15q Clinical Research Network, the Angelman Syndrome Foundation, the Dup15q Alliance as well as several other research studies to collect and integrate datasets. The datasets that are shared with LADDER are de-identified, which means we do not know who the data is about. By signing up with LADDER you provide consent for us to use the information you provide during enrollment (e.g. date of birth, information about the diagnosis) in order to identify and link the existing data in LADDER. So, for example, if you have previously participated in the AS Natural History study, registered with the ASF and gone to an AS clinic, we will be able to link the data from these different programs into one set of information on your child. The identifiable information you provide will not be shared outside of LADDER unless we have your explicit consent to share it.
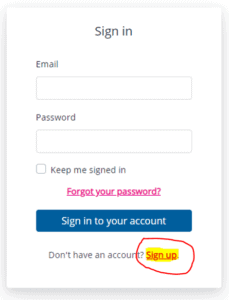
- Go to laddertotreatment.com/user/register and click Sign up.
2. Complete the Create your LADDER account form.
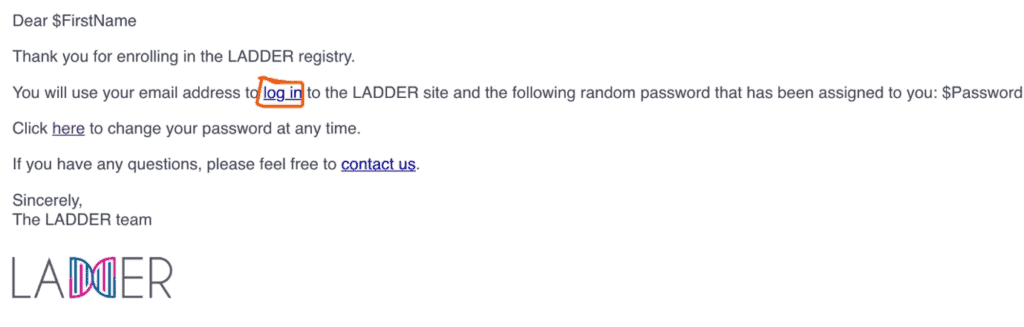
3. You will then receive an email at the address you entered. Open the email and click the login link in the email to be returned to the portal to change your password.
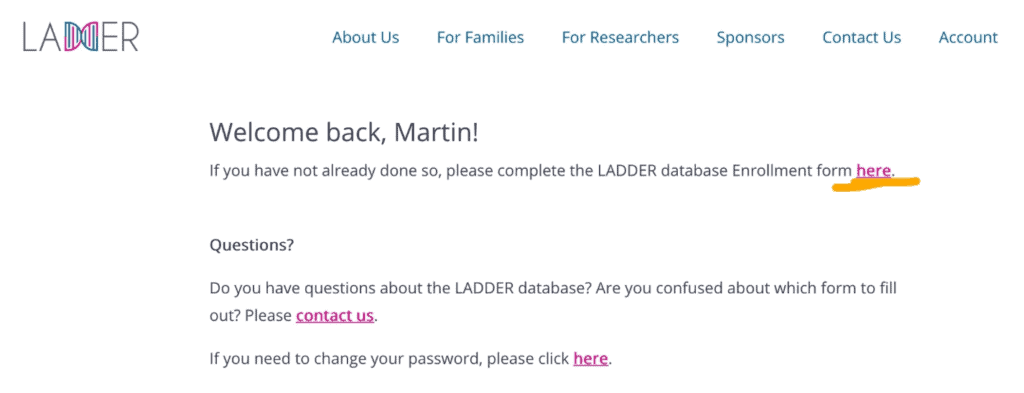
4. Return to your account page laddertotreatment.org/user/account and sign in.
5. On the Welcome back screen, click to complete the Enrollment form.
5. After completing the form, you are done!