Research is key to improving the lives of individuals with Angelman syndrome and to finding a cure. In recent years, there have been critical discoveries that have greatly improved our understanding of Angelman syndrome and lead to critical discoveries, clinical trials and increased funding by the NIH.
But we’re not slowing down! The ASF continues to push forward to find new and innovative ideas.
Below, find information about the ASF’s approach to funding research and how it has impacted AS.
Twice a year, scientists from all over the world submit requests for funding. The ASF Scientific Advisory Committee selects the most innovative, promising and most likely to impact the lives of individuals with AS.
Some project ideas are great, but there is little data to prove it. The ASF provides pilot funding. With pilot funding, researchers can prove their idea is solid. Then, funding at much higher amounts are granted by the NIH or pharma.
![]()
Dr. Zylka explains pilot funding.
Examples:
NIH invests $2.8 million
NIH invested $2.1 million
NIH invests $2 million
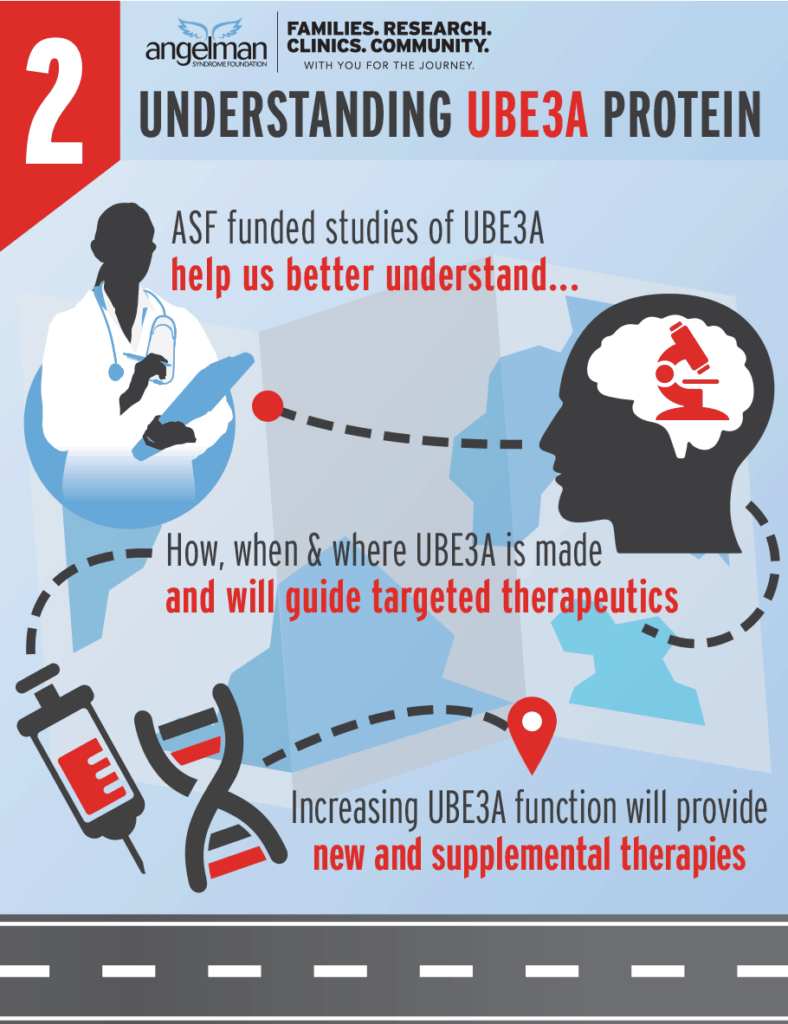
The ASF funds research to help understand UBE3A.
Information learned from many different ASF funded studies helped contribute to the many different approaches for therapies.
Therapies are available because ASF invested in projects to understand UBE3A and how it is regulated. We will continue to invest in this vital knowledge because it will lead to other potential therapies.
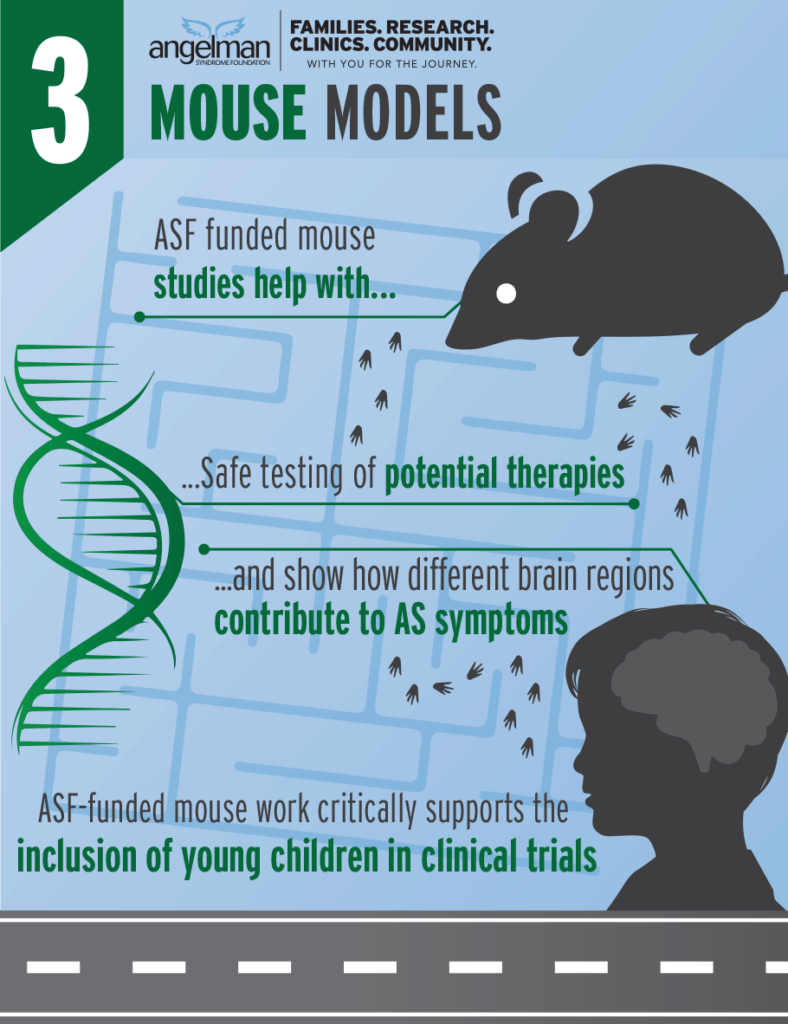
ASF has funded several different mouse models of AS. These models have been instrumental for studies of therapeutic approaches and for understanding the brain dysfunction caused by AS.
ASF-funded mouse studies proved that earlier restoration of UBE3A would give better outcomes following genetic therapies.
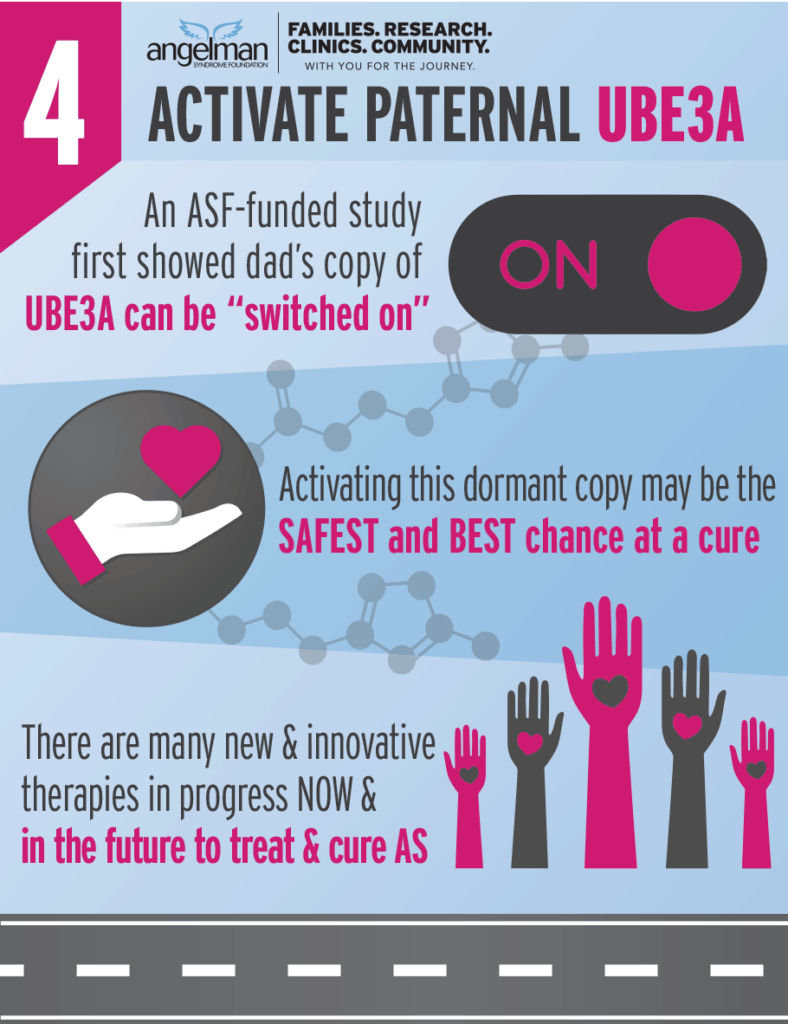
ASF funded the very first studies that proved that paternal UBE3A can be activated.
ASF funded the first ASO studies proving that the therapeutic approach would work.
ASF has also funded new, innovative ideas still in the development stage but strong enough to be supported by pharmaceutical companies.
We will keep pushing forward with new and better therapeutic ideas.
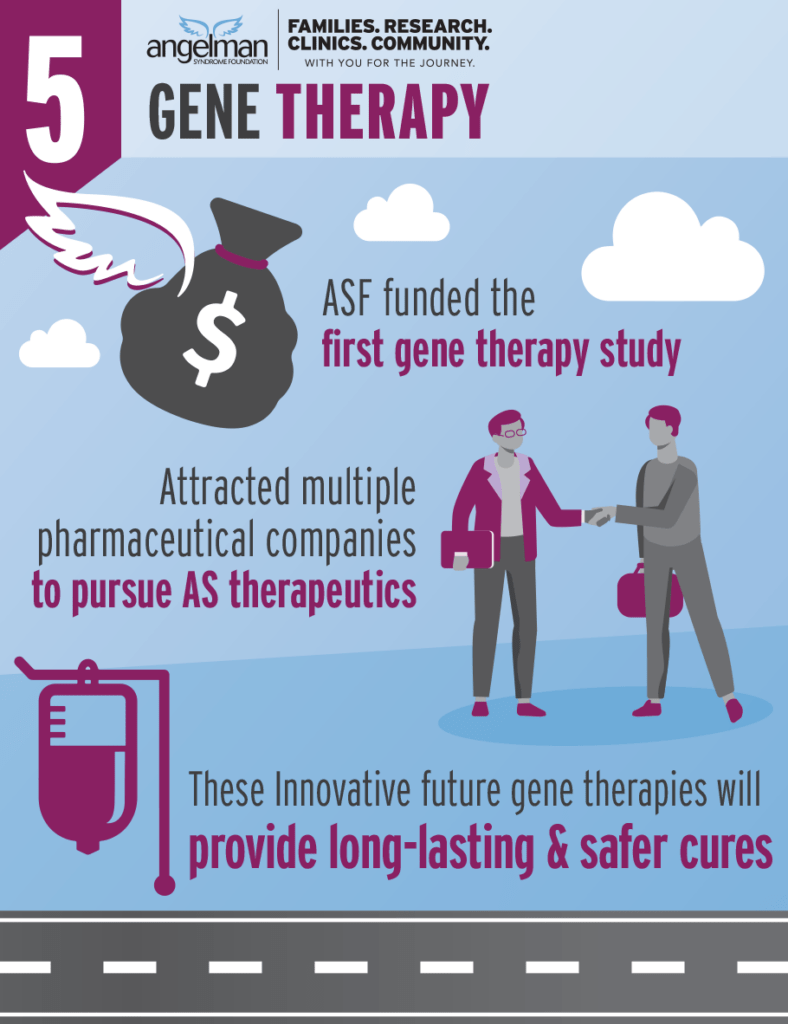
ASF funded the first gene therapy study and has funded two more innovative twists to make an optimal gene therapy.
These studies along with the gene activation strategies (UBE3A activation and ASOs) have attracted several pharmaceutical companies to develop potential therapies for AS.
Examples:
OVID working with UCONN
Therapies and therapeutics CANNOT move forward without a substantial amount of knowledge about individuals with AS.
To support this need, ASF funds a global network of clinics and worked with partners to establish the LADDER database.
The network of clinics serves patients across the globe and the LADDER database collects information from various sources – all to help us better understand AS and inform the best design for clinical trials.
We are better together!
The partnership between expert clinicians, brilliant scientists and a dedicated Angelman community has resulted in exciting clinical trials- with more to come!
Partnerships with other patient advocacy groups like FAST, CASS, ASA, the Dup15q Alliance and Angelman Argentina have enriched our knowledgebase and expanded our impact.
We hope you continue on this journey with the ASF!
Details on ASF-Funded Research
Visit ASF Funded Research to see all research projects funded by the ASF and the Research Funding Philosophy to find out more about how our Scientific Advisory Committee prioritizes studies to fund.
Published Research on Angelman Syndrome
The United States National Library of Medicine at the National Institutes of Health maintains a database of completed research on life sciences and biomedical topics. Here you can find links to full text articles of all published research that has been completed on Angelman syndrome.